All of the Following Compounds Are Soluble in Water Except:
The incorporation of carbon dioxide into organic compounds is known as carbon fixation. All the water-soluble vitamins are easy to get from a balanced diet.
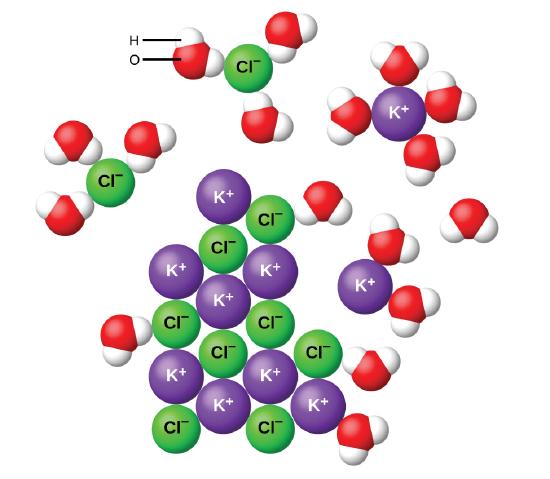
7 5 Aqueous Solutions And Solubility Compounds Dissolved In Water Chemistry Libretexts
Salts can be divided into two types.
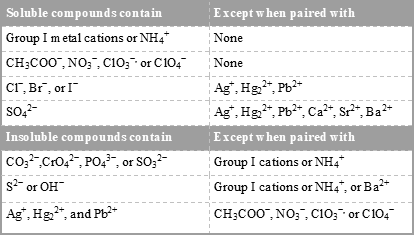
. The extent of the solubility of a substance in a specific solvent is generally measured as the concentration of the solute in a saturated solution one in which no more solute can. In chemistry ironIII refers to the element iron in its 3 oxidation stateIn ionic compounds salts such an atom may occur as a separate cation positive ion denoted by Fe 3. Select all that apply.
For example AgNO 3 is water-soluble but AgCl is water-insoluble. -oxidation-reduction and synthesis -oxidation-reduction only -synthesis. As a result vegans are at a high risk of.
Iron and manganese in well waters occur as soluble ferrous and manganous bicarbonates. Iron and Manganese Removal. B A net ionic equation consists of the formulas for the spectator ions.
Viscosity of poloxamers depends on the increasing percentages of polyoxypropylene hydrophobe and polyoxyethylene hydrophile. The solubility of ionic compounds in water depends on the type of ions cation and anion that form the compounds. The macromolecular components of all enzymes consist of protein except in the class of RNA catalysts called ribozymes.
A BaSiF 6 0026 g100 mL contains latextextSiF_62-latex ions. Air pollution standards must be considered when air stripping is used to reduce volatile organic compounds. The Handbook of Chemistry and Physics gives solubilities of the following compounds in grams per 100 mL of water.
A All species are represented as ions in this type of equation. Because these compounds are only slightly soluble assume that the volume does not change on dissolution and calculate the solubility product for each. Insolubility is the opposite property the inability of the solute to form such a solution.
On d 14 rats were gavaged with 0 00375 005 0075 or 015 mL CCl4kg and hepatic and renal toxicity assessed 24 hr later. Those soluble in water and those insoluble in water. Which of the following statements correctly describe a net ionic equation.
The word ribozyme is derived from the ribonucleic acid enzyme. The energy for this comes from the first phase of the photosynthetic process. -NaCl - CaCl2 -FeCl3 -BaCl2 - PbCl2 The equation 2Als 3Br2l 2AlBr3s is an _____ reaction.
However vitamin B12 is only found in substantial amounts in animal-sourced foods. Phenol C 6 H 6 OH is a solid in winter and liquid in summer mp. 4FeHCO 3 2 O 2 2H 2 O 4FeOH 3- 8CO 2.
Little or no hepatic and renal toxicity was observed in. Poloxamers are water soluble and can form gels in a concentrated aqueous solution which is reversible to liquid form after lowering the temperature and vice versa. The adjective ferric or the prefix ferri- is often used to specify such compounds as in ferric chloride for ironIII chloride FeCl 3The adjective ferrous is used instead for ironII salts containing.
Enzymes are found in all tissues and fluids of the body. Many ribozymes are molecules of ribonucleic acid which catalyze reactions in one of their own bonds or among other RNAs. Start studying the chem quiz 7 flashcards containing study terms like Which of the following ionic compounds is insoluble in water.
Because exposure to the 25-chemical mixture via the drinking water resulted in decreased water and feed consumption restricted deionized water and feed controls Restricted Water were included. All are colourless when pure but generally slightly coloured due to oxidation. You should know some simple solubiity rules which will allow you to know which salts are soluble in water.
A compound is probably soluble if it contains one of the following cations. In chemistry solubility is the ability of a substance the solute to form a solution with another substance the solvent. Memorize flashcards and build a practice test to quiz yourself before your exam.
All except m-cresol are solids m-cresol is a liquid. Living systems cannot directly utilize light energy but can through a complicated series of reactions convert it into C-C bond energy that can be released by glycolysis and other metabolic processes. C A net ionic equation shows only the chemical change that is taking place in a reaction.
O m and p-cresol catechol and resorcinol. As mentioned PEGPPG-176 copolymer could be under the umbrella of alkyl PEG. Li Na K Rb Cs Ammonium.
In the aeration process the water is saturated with oxygen to promote the following reactions. They have low solubility in water but have appreciable solubility in. McMurry and Fay give two basic solubility rules.
The solubility of a salt can be predicted by following a set of empirical rules listed below developed based on the observations on many ionic compounds. Other examples are.

Question Video Using The Water Solubility Rules To Determine Which Chloride Compound Is Insoluble Nagwa

Solubility Rules Chart Chemistry Chemtalk
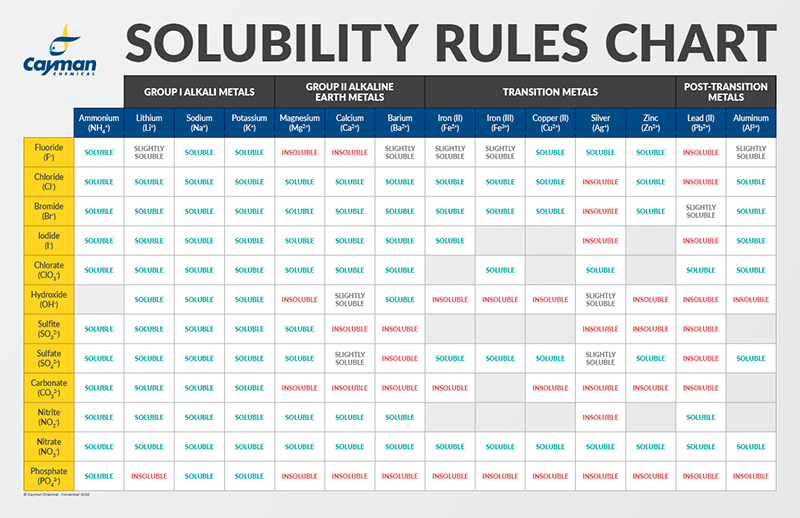
Solubility Factors When Choosing A Solvent News Announcements Cayman Chemical
Comments
Post a Comment